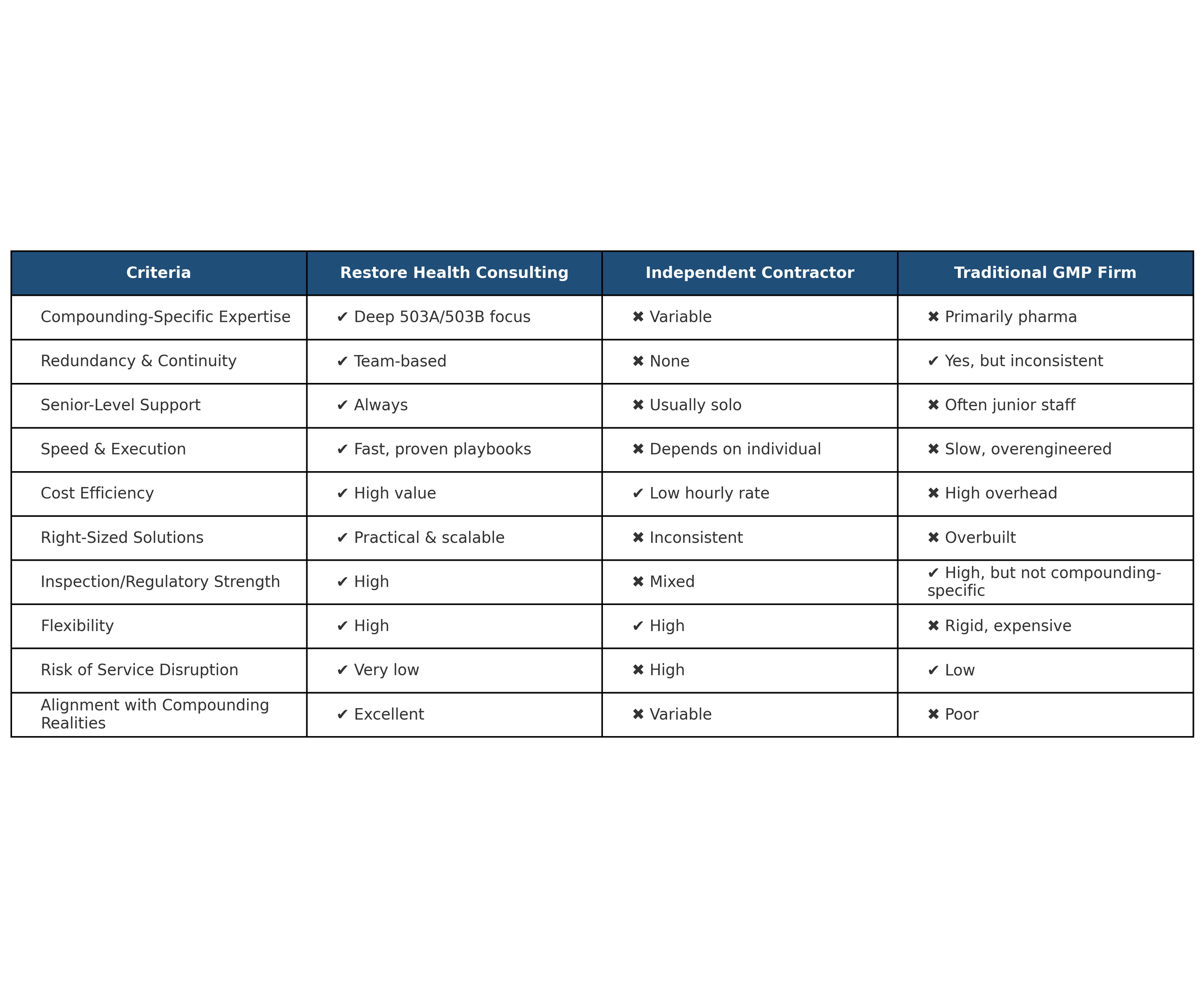
Why RHC
RHC hits the industry’s sweet spot—practical, agile, and highly experienced.
We offer more depth than a single contractor and more flexibility than large pharma-focused consultancy firms, with expertise built for today’s compounding and personalized medicine space.